Ternary lithium batteries, also known as NCM batteries, are a type of rechargeable battery that has garnered significant attention due to their high energy density, long lifespan, and robust performance. This guide delves into the characteristics and manufacturing methods of the cathode material—Nickel Cobalt Manganese Oxide (NCM)—and the anode material—graphite. Understanding these materials is crucial for appreciating the advantages and applications of ternary lithium batteries.
1. Introduction
Ternary lithium batteries have become a staple in modern energy storage solutions, powering everything from electric vehicles (EVs) to portable electronics. The key components of these batteries are the cathode and anode materials. This guide will explore the characteristics and preparation methods of Nickel Cobalt Manganese Oxide (NCM) as the cathode and graphite as the anode.
2. Characteristics of NCM (Nickel Cobalt Manganese Oxide)
Chemical Composition
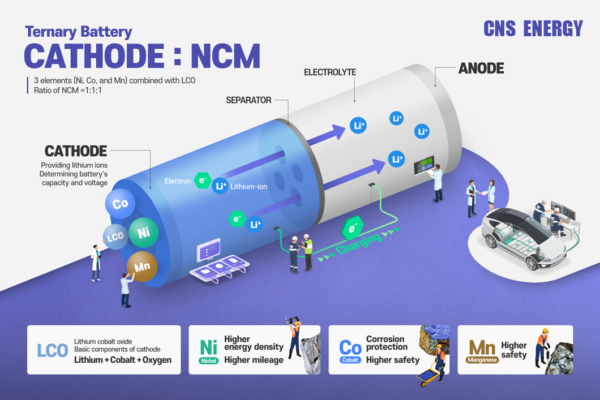
Nickel Cobalt Manganese Oxide (LiNiCoMnO2) is a complex compound that integrates the benefits of nickel (Ni), cobalt (Co), and manganese (Mn). Each element contributes uniquely to the performance of the cathode material:
Nickel: Increases energy density and capacity.
Cobalt: Stabilizes the structure and enhances safety.
Manganese: Improves thermal stability and reduces costs.
Properties
High Energy Density: NCM offers a high specific capacity, making it ideal for applications requiring long battery life.
Thermal Stability: The presence of manganese improves thermal stability, reducing the risk of overheating.
Long Cycle Life: NCM materials are known for their ability to maintain capacity over many charge and discharge cycles.
Safety: The balanced composition provides a safer alternative compared to other high-energy-density materials.
3. Preparation Methods of NCM
Co-precipitation Method
The co-precipitation method is widely used for synthesizing NCM. This process involves the following steps:
Precipitation: Nickel, cobalt, and manganese salts are dissolved in water. A precipitating agent, usually sodium hydroxide (NaOH), is added to form hydroxides.
Mixing: The resulting hydroxides are mixed with a lithium source, typically lithium carbonate (Li2CO3).
Calcination: The mixture is heated at high temperatures (700-1000°C) to form the NCM compound.
Sol-Gel Method
This method involves the formation of a gel from a solution and subsequent thermal treatment:
Solution Preparation: Metal salts and a lithium source are dissolved in a solvent.
Gel Formation: A chelating agent, such as citric acid, is added to form a gel.
Drying and Calcination: The gel is dried and then calcined to obtain NCM powder.
4. Characteristics of Graphite
Chemical Composition
Graphite, a form of carbon, is the most commonly used anode material in lithium-ion batteries. Its layered structure allows for the intercalation and de-intercalation of lithium ions during charge and discharge cycles.
Properties
High Electrical Conductivity: Graphite’s excellent conductivity enhances the battery’s overall performance.
Lithium Intercalation: The layered structure of graphite accommodates lithium ions, contributing to high capacity and efficiency.
Stability: Graphite anodes exhibit good structural stability and low volume change during cycling, which prolongs battery life.
Abundance and Cost-Effectiveness: Graphite is abundant and relatively inexpensive, making it a cost-effective choice for battery manufacturers.
5. Preparation Methods of Graphite
Natural Graphite
Natural graphite is mined and then processed to enhance its suitability for battery applications:
Purification: Impurities are removed through chemical or thermal treatments.
Spheronization: Graphite flakes are milled into spherical particles to improve packing density.
Coating: Surface treatments are applied to enhance performance and compatibility with electrolytes.
Synthetic Graphite
Synthetic graphite is produced from petroleum coke through high-temperature treatment:
Carbonization: Petroleum coke is heated to form carbon.
Graphitization: The carbonized material is further heated to 3000°C to transform it into graphite.
Purification and Shaping: Similar to natural graphite, synthetic graphite undergoes purification and shaping processes.
6. Comparative Analysis
Energy Density
NCM: Provides higher energy density compared to other cathode materials.
Graphite: Offers good capacity but lower energy density compared to NCM.
Stability and Safety
NCM: Improved thermal stability with manganese; cobalt adds safety.
Graphite: Excellent structural stability and low volume change during cycling.
Cost
NCM: Generally more expensive due to cobalt content.
Graphite: More cost-effective, especially natural graphite.
7. Applications and Benefits
Electric Vehicles (EVs)
NCM: High energy density makes it ideal for long-range EVs.
Graphite: Provides stable performance and longevity for EV batteries.
Portable Electronics
NCM: Powers smartphones, laptops, and tablets with high capacity and reliability.
Graphite: Ensures efficient charge and discharge cycles, prolonging device life.
Energy Storage Systems (ESS)
NCM: Suitable for large-scale energy storage due to high capacity and efficiency.
Graphite: Contributes to the overall stability and longevity of ESS.
8. Conclusion
Understanding the characteristics and preparation methods of NCM and graphite is essential for leveraging their advantages in ternary lithium batteries. NCM’s high energy density and stability, combined with graphite’s conductivity and structural integrity, create a powerful synergy that drives the performance of modern rechargeable batteries.
9. References
“Nickel Cobalt Manganese Oxide (NCM) Synthesis and Applications,” Journal of Materials Chemistry, 2021.
“Graphite Anodes for Lithium-Ion Batteries,” Electrochemical Society Interface, 2020.
“Advanced Manufacturing Processes for Battery Materials,” Journal of Power Sources, 2019.
By understanding these materials and their preparation methods, industry professionals can optimize battery design and performance, ensuring the continued growth and success of applications powered by ternary lithium batteries.